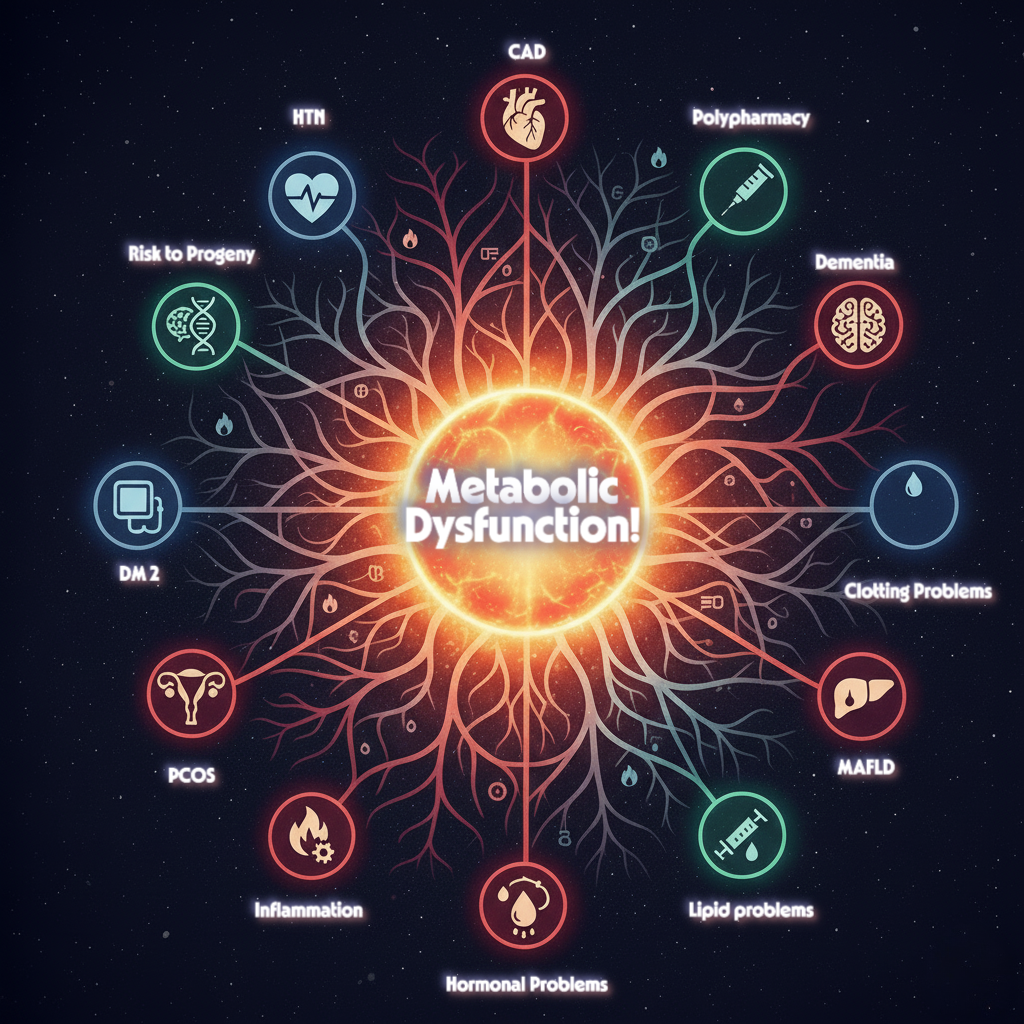
We often view chronic conditions like Type 2 Diabetes, Cardiovascular Disease (CVD), Polycystic Ovary Syndrome (PCOS), and even Alzheimer’s as separate health battles, each requiring a different specialist. However, cutting-edge research reveals a startling and unifying truth: this seemingly disparate list of ailments—which also includes Metabolic Syndrome, NAFLD/MASLD, and Hypertension—shares a common, dangerous foundation in metabolic dysfunction.
This paradigm shift moves the focus from treating isolated symptoms to tackling the shared molecular roots, offering a powerful blueprint for integrated management and preventative care.
Shared Pathophysiology: A Vicious Cycle of Dysfunction
At the core of this unified theory are three main drivers: Chronic Insulin Resistance, Visceral Adiposity, and Chronic Low-Grade Inflammation.
1. The Insulin Resistance Bridge
Insulin resistance, the body’s decreased response to insulin, is the central pillar of this metabolic web. It is driven primarily by excessive visceral adiposity—fat stored around abdominal organs—which is far more metabolically active than subcutaneous fat.
- In T2DM, insulin resistance prevents muscle and fat cells from efficiently taking up glucose, leading to hyperglycemia.
- In PCOS, hyperinsulinemia disrupts ovarian function and androgen metabolism.
- Perhaps most surprising is the link to Alzheimer’s disease, which is increasingly termed “Type 3 Diabetes.” In the brain, insulin resistance impairs neuronal function, synaptic plasticity, and the clearance of harmful amyloid-beta, directly contributing to cognitive decline.
2. Adipokines and the Inflammatory Cascade
Visceral fat is not merely an inert storage depot; it is a highly active endocrine organ. Hypertrophied adipose tissue releases a toxic cocktail of pro-inflammatory messengers called adipokines (like leptin and resistin) and inflammatory cytokines (like TNF-α and IL-6).
This chronic, low-grade inflammation is the molecular bridge that drives pathology across multiple organ systems:
- It promotes endothelial dysfunction and atherogenesis in the blood vessels, leading directly to CVD and Hypertension.
- It impairs liver fat metabolism, leading to hepatic steatosis in NAFLD/MASLD.
- It exacerbates insulin resistance systemically, creating a devastating feedback loop that fuels the progression of nearly every condition on the list.
From Individual Treatment to Integrated Management
Understanding the common molecular pathways—the interplay of dysregulated adipokine signaling, impaired insulin function, and systemic inflammation—is key to developing unifying therapeutic strategies.
The Cornerstone: Lifestyle Intervention
The most potent and broadly applicable therapy for managing and preventing all these conditions remains intensive lifestyle modification. The evidence is robust: achieving and maintaining just 7–10% weight loss combined with ≥150 minutes per week of moderate-intensity physical activity is the gold standard. Diet, particularly the Mediterranean diet or similar reduced-calorie approaches, provides the essential fuel for metabolic repair. Sometimes, complexity and intricate protocols are not the best, easy interventions like what you eat, how much you move can make all the difference and not getting sick is a better option than trying to find a remedy which may or may not work.
Targeting the Metabolic Roots with Pharmacotherapy
Modern medicine is increasingly targeting the underlying metabolic roots, offering multi-system benefits that go beyond simple glucose or blood pressure control.
- GLP-1 Receptor Agonists (e.g., semaglutide, liraglutide) and Tirzepatide offer significant advantages in glycemic control, sustained weight reduction, and proven cardiovascular protection. They act as powerful metabolic modulators, improving insulin sensitivity and reducing overall cardiometabolic risk.
- SGLT2 Inhibitors also provide robust cardiovascular and renal benefits.
These agents represent a major step forward, validating the strategy of treating the underlying metabolic condition rather than just the resultant disease. As research continues, particularly in establishing definitive preventative strategies for Alzheimer’s disease, the future of chronic care lies in this integrated, metabolic first approach.